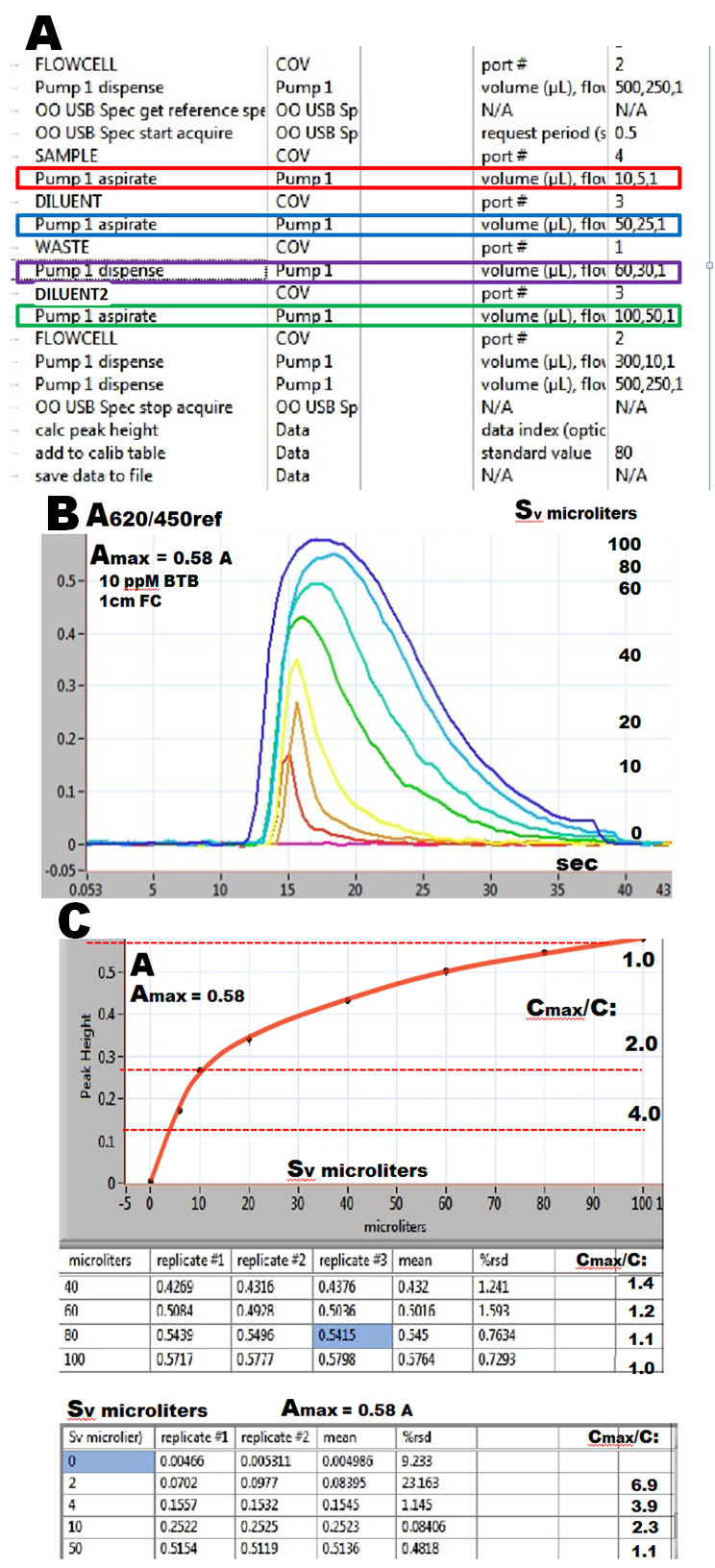
Degree of dilution and its reproducibility discussed in this, and the following sections was determined by monitoring the absorbance of samples of bromothymol blue (BTB), which were diluted by carrier stream and by defined volumes of diluent (Dv and D2v). The carrier solution and diluent were identical in composition, being buffered to pH 9.3 ( 0.001 M borax buffer) to prevent changes of BTB absorbance due to pH fluctuations. The instrument, miniSIA-1 was configured as shown on the flow scheme on the previous page. Assay protocol (A) specifies the sequence, volumes and flow rates for all four steps of the Sequential Dilution protocol.
Sample volume (Sv), the first variable of the dilution protocol was investigated by means of the assay protocol where the values of the other variables (Dv, Cv and D2v blue, mauve and green box) were set to zero. As sample solution 10ppM BTB was used and absorbancies for Sv from 0 to 100 microliters were recorded (B) and plotted (C). The absorbance of undiluted dye (Amax = 0.58) was measured when the flow cell ( light path 1cm) was filled with undiluted dye (Cmax/C=1)
It follows that decreasing the volume of injected sample offers only a limited degree of dilution, up to about 4 times, since volumes below Sv= 4 microliters can not be reproducibly metered. Therefore all following experiment were done using Sv of 10 microliters. Note that the value of Amax=0.58 obtained for 10ppM BTB will be used for extrapolation of Amax for 100ppM and 1000ppM BTB solutions used in next experiments.
Sequential Dilution for Sample Preparation
Sample Injection
(Dilution 0 to 7x)
2.2.6.H.